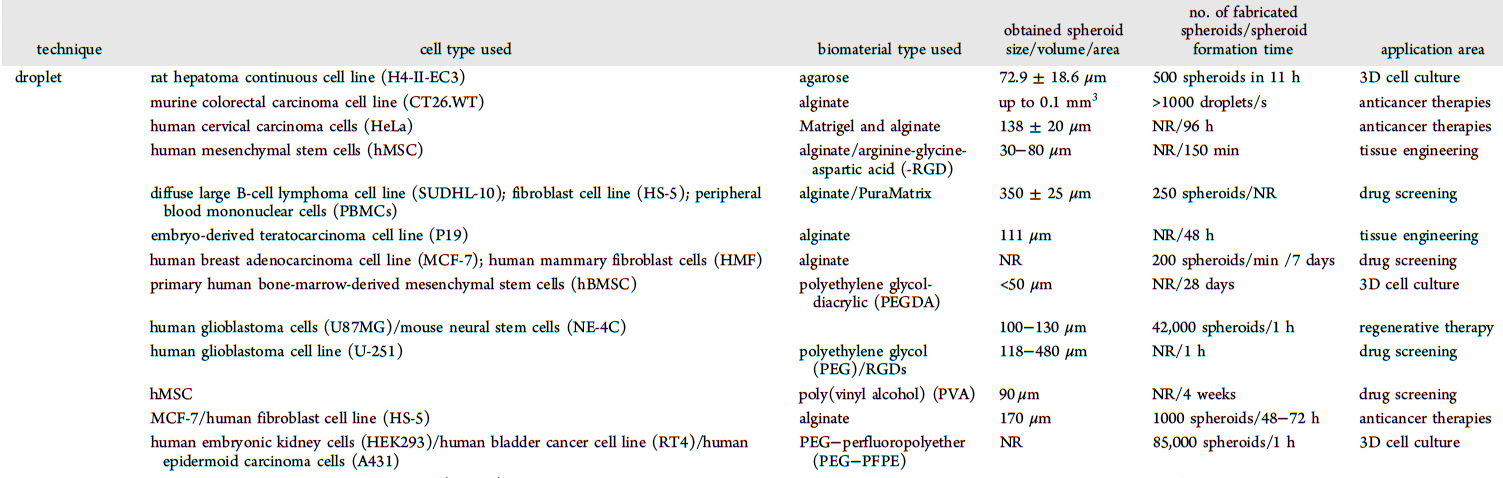
Spheroid engineering in microfluidic droplets is an emerging area of research that combines microfluidic technology with tissue engineering principles to create 3D cell cultures. Spheroids are aggregates of cells that are cultured in vitro to mimic the in vivo behavior of tissues. In comparison to 2D cell culture methods, 3D cell cultures such as spheroids enable the equal distribution of nutrients, gases, and growth factors among cells and the surrounding extracellular matrix. Spheroids also promote the investigation of cell-to-cell interactions and the cellular microenvironment, which cannot be accurately represented in 2D culture. While conventional approaches to spheroid fabrication have demonstrated the utility of 3D cell cultures to applications such as disease modeling and drug development, these techniques are labor-intensive, limited in throughput, and often result in irregular spheroids that limit reproducibility. As a result, spheroid engineering in microfluidic droplets is becoming an increasingly popular technique, as it promotes the production of homogenous spheroids with high throughput and precision. The integration of microfluidics technology with tissue engineering strategies enables robust organ-on-a-chip applications in disease research, disease modeling, and drug discovery.
 T-junction geometry (2) co-flowing and (3) flow focusing.png)
The process of creating spheroids in microfluidic droplets typically involves three main steps: droplet generation, cell encapsulation, and culture. First, droplets of a specific size and shape are generated using microfluidic channels or nozzles. Then, 20-200 cells are typically encapsulated within droplets. Finally, the droplets containing cells are cultured under specific conditions to allow the cells to aggregate and form spheroids. Generally, clustered cells form tightly packed 3D spheres with flattened cells forming the outermost boundaries and the inner core containing cells with rounded and polygonal shapes. However, other shapes like mass and grape-like structures have also been observed. In the case of spheroids made with cancer cells, the structure comprises three distinct areas: an outer layer consisting of actively proliferating and migrating cells, a middle layer of senescent cells, and a necrotic cell core. This organization mimics the tumor microenvironment, where proliferating cells are located close to blood vessels, and inner cells remain quiescent. For drug screening, cancer spheroids are typically 200 µm in size. However, larger spheroids are more phenotypically similar to tumor xenografts. The shape, mechanical properties, and phenotype of spheroids can be altered by incorporating extracellular matrix (ECM) proteins or gels inside or around them.
Key benefits of generating spheroids in microfluidic droplets include the ability to control spheroid size and shape and to conduct high-throughput experiments. In drug discovery applications, the droplet format of spheroids offers an advantage in generating many biological replicates in a single run. This enables high-throughput measurements of functional behaviors in individual droplets. In addition to end-point measurements of cells and supernatants, cell fate and behavior can be continuously monitored in droplets. Live microscopy has been widely used to dynamically monitor cell behavior in droplets, enabling real-time imaging of stress responses, protein secretion, cell-cell interactions, and the acquisition of differentiated or activated phenotypes. Additionally, live/dead assays have been employed to quantify viability in droplets, especially for assessing drug toxicity and resistance of spheroids over periods ranging from a few hours to a couple of days. To do this, reagents such as CellTrace or Hoechst for staining total cells, calcein AM for live cells, and propidium iodide (PI) for labeling dead cells are mixed with the cells prior to loading them into droplets. The droplets are then individually imaged at frequencies ranging from minutes to several hours.
In addition to drug screening, spheroids have been used to study the behavior of immune cells in cancer biology. Matrigel droplets containing a single spheroid were used to study the search and recruitment dynamics of GFP+ T cells as they interacted with each cancer spheroid. The interactions between the T cells and the cancer spheroids were observed, yielding a large number of cell-cell interactions. T-cell-mediated killing of tumor cells was tracked by examining the apoptotic expression of caspase 3/7. The results revealed that T cells target the killing of tumor cells in two distinct mechanisms: a positive-feedback loop driving T cell accumulation on cancer spheroids and local cooperative killing of T cells on cancer spheroids. This study replicated intricate immune cell behavior previously observed in vivo while taking advantage of the high-throughput capacity of droplet microfluidics. Other studies of cancer biology using droplet-generated spheroids have measured cell proliferation, the cytotoxic effects of anticancer drugs, and drug resistance. Spheroids have also been used to model Alzheimer’s disease, non-alcoholic fatty liver disease, and diabetes mellitus. As organ-on-a-chip technologies such as spheroid engineering continue to mature, the ability to effortlessly and reliably generate droplets of a precise diameter becomes paramount. Learn more about the Onyx droplet generator here.
- Sart S, Ronteix G, Jain S, Amselem G, Baroud CN. Cell Culture in Microfluidic Droplets. Chem Rev. 2022 Apr 13;122(7):7061-7096. doi: 10.1021/acs.chemrev.1c00666. Epub 2022 Feb 18. PMID: 35179881.
- Tevlek A, Kecili S, Ozcelik OS, Kulah H, Tekin HC. Spheroid Engineering in Microfluidic Devices. ACS Omega. 2023 Jan 18;8(4):3630-3649. doi: 10.1021/acsomega.2c06052. PMID: 36743071; PMCID: PMC9893254.


